
Aditya Raj Anand
Thursday 24 September 2020
Chemistry
In this post we discuss 10 key difference between electrolytic cell and electrochemical cell. also understand the concept of electrochemistry. How electrochemistry comes? What is electrochemistry?
Electrolytic cell and electrochemical cell both are interconnected to the chapter Electrochemistry. How? we discuss later. Also both have many differences between them. Here we learn not only the concept of electrolytic cell and electrochemical cell but also try to understand the how these cells are created.
But before we discuss both these cells. we have to understand the term electrochemistry. How the concept of electrochemistry comes as a chapter in chemistry? In simple word electrochemistry means electricity from chemical reaction or chemical reaction from electricity.
10 key difference between electrolytic cell and electrochemical cell
In simple line the basic difference is electrolytic cell are those cells in which electrical energy is used to carry out non-spontaneous chemical reaction on the other hand electrochemical cells are those cells in which chemical energy is is used to carry out spontaneous reaction to form electricity.
Here is 10 key difference between electrolytic cell and electrochemical cell to clear your concept.
Electrolytic cell and Electrochemical cell (or Galvanic cell):-
(1.) Electrolytic cell converts electrical energy into chemical energy whereas Galvanic cell converts chemical energy into electrical energy.
(2.) Electrolytic cell carry out non-spontaneous chemical reaction whereas Galvanic cell carry out spontaneous chemical reaction.
(3.) In an electrolytic cell, external source of voltage is used to bring out a chemical reaction whereas In electrochemical cell (or galvanic cell) no external source of voltage is required to bring out chemical reaction.
(4.) In electrolytic cell, Oxidation occurs at cathode whereas In galvanic cell Oxidation occurs at anode.
(5.) In electrolytic cell, Reduction occurs at anode whereas In galvanic cell reduction occurs at cathode.
(6.) In electrolytic cell, cathode is negatively charge whereas In galvanic cell cathode is positively charge.
(7.) In electrolytic cell, anode is positively charge whereas In galvanic cell anode is negatively charge.
(8.) In electrolytic cell, Both rods anode and cathode are in the same beakers whereas In galvanic cell both rods anode and cathode are in different beakers.
(9.) In electrolytic cell there is no salt bridge present but In galvanic cell there is salt bridge present.
(10.) In electrolytic cell, there is only one metal electrode whereas In galvanic cell there is two different types of metal electrode.
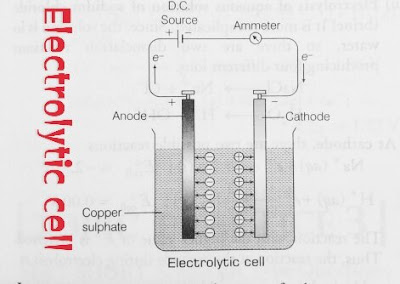
What is Electrolytic Cell?
Electrolytic cells are those cells in which electrical energy converted into chemical energy. it is non-spontaneous chemical reaction.
In an electrolytic cell, external source DC is used to bring about a chemical reaction. In above electrolytic cell diagram. electrolytic cell consists of two copper electrode. The two copper electrode acts as anode and cathode dipped in copper sulphate solution. When DC voltage is supplied in the two copper electrode then positive charge anode acts as cathode and negatively charge cathode acts as anode. As we discuss earlier, In an electrolytic cell oxidation occurs at anode whereas reduction occurs at cathode. so we can see it in the above diagram anode loss electron (oxidation) on the other hand cathode gain electron (reduction).
In industrial process, impure copper rod is acts as an anode but pure copper rod acts as cathode at which cu2+ ions deposits.
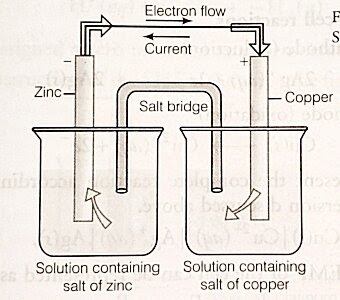
What is Electrochemical cell (or Galvanic cell)?
Electrochemical cells are those cells which convert chemical energy into electrical energy through a spontaneous redox reaction. It is also know as galvanic or voltaic cell. Because galvanic cell also convert chemical energy into electrical energy.
Electrochemical cell are two types Galvanic cell (further after improvement known as Daniell cell) and Electrolytic cell. examples of electrochemical cells are remote battery, car battery, acid battery etc.
Galvanic cells consists of two half-cells, each having different salt solution. basically galvanic cell has two container. Each container has one metal electrode connected with other metal electrode present on other container. Both the container are linked with salt bridge.
The electrodes present on both the container are connected with conducting wire. Due to these connecting wire Oxidation and reduction takes place in different half-cell. Which result in the development of potential difference between the two half-cells and hence, electricity generated.
See the diagram of galvanic cell for better understanding.
How to determine anode and cathode in galvanic cell
In Galvanic cell, an electrode is dipped into the solution of its own salt, this combination is called half-cell or redox couple. In a half-cell, either a reduction or an oxidation reaction occurs. But Oxidation occurs at anode whereas reduction occurs at the cathode. So we can easily determined anode and cathode just looking the occurrence of oxidation and reduction.


This is better
ReplyDeleteThank You, I hope you love the information. The main reason of the author of this website is to clear the doubt of every students in Physics and chemistry. If you want to support us in any way, please buy our first book on physics. available on Amazon or Flipkart. Just type there Aditya Raj Anand. you will get the all books written by me.
ReplyDelete